Unsung helper that protects cells from mechanical stress holds the therapeutic promise
Unsung helper that protects cells from mechanical stress holds the therapeutic promise
A hidden protein that protects our cells from mechanical stress could provide a new direction to therapeutic strategies for diseases where protein stability under force is compromised like heart muscle disease or genetic disorders called laminopathies.
In the dynamic environment of a living cell, proteins are constantly pulled, pushed, and twisted by molecular forces generated during essential processes such as transport, degradation, and cytoskeletal remodeling. These mechanical stresses influence how proteins fold, unfold, and perform their functions. While much attention has been paid to specialized proteins called canonical chaperones that guide folding, scientists have been exploring whether their accessory cofactors can directly help proteins withstand physical forces.
A recent study by researchers at the S. N. Bose National Centre for Basic Sciences (SNBNCBS), an autonomous institute of Department of Science & Technology (DST), led by Dr. Shubhasis Haldar, sheds new light on this question. Their work has revealed an unexpected hero in the mechanical stability landscape: p47, a cofactor protein usually known as a helper for the cellular machine p97. While p97 is a powerhouse involved in moving and degrading proteins, p47 was long thought to be just an assistant known primarily for its role in protein trafficking, degradation, and membrane fusion.
Using single-molecule magnetic tweezers, the team applied controlled mechanical forces to individual protein molecules, mimicking the kinds of physical strains proteins encounter during processes like transport within the cell or stretching by the cell’s internal framework
Remarkably, they found that p47 is not just a passive helper for p97, it can directly stabilize proteins under force, effectively acting as a “mechanical chaperone.” In the experiments, p47 bound to mechanically stretched proteins and enhanced their ability to refold, even under constant pulling forces. This mechanical assistance mirrors the foldase-like activity of canonical chaperones but comes from an accessory factor that was not previously thought to possess such a function.
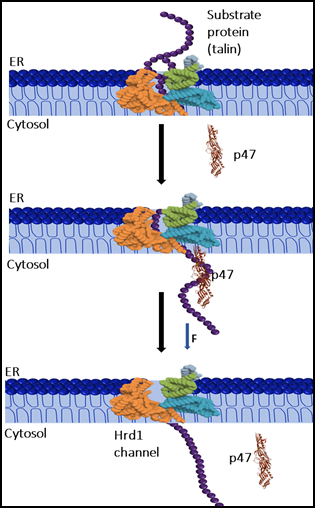
Fig: p47 participates in the mechanical extraction of proteins from the ER lumen to the cytoplasm. By exhibiting chaperone-like properties, p47 enhances mechanical efficiency and facilitates polypeptide translocation through the pore.
The study provides direct, single-molecule evidence that cofactors like p47 can have autonomous, force-dependent protective activity. This discovery opens the door to rethinking the broader roles of accessory proteins in cellular mechanics and protein quality control.
The findings, published in Biochemistry as part of a special issue celebrating the 25th Anniversary of the Chemical Research Society of India, expand the functional repertoire of accessory proteins. They suggest that targeting mechanical cofactors like p47 could be a new route in therapeutic strategies for diseases where protein stability under force is compromised.
***